For people living with autoimmune diseases like rheumatoid arthritis, psoriatic arthritis, or Crohn’s disease, pain and fatigue aren’t just daily inconveniences-they’re life-shaping realities. For decades, treatments were limited to drugs that eased symptoms but couldn’t stop the damage. Then came TNF inhibitors. These aren’t your average pills. They’re precision-engineered biologics that target a single molecule: tumor necrosis factor alpha, or TNFα. And for many, they’ve turned impossible days into manageable ones.
What Is TNFα, and Why Does It Matter?
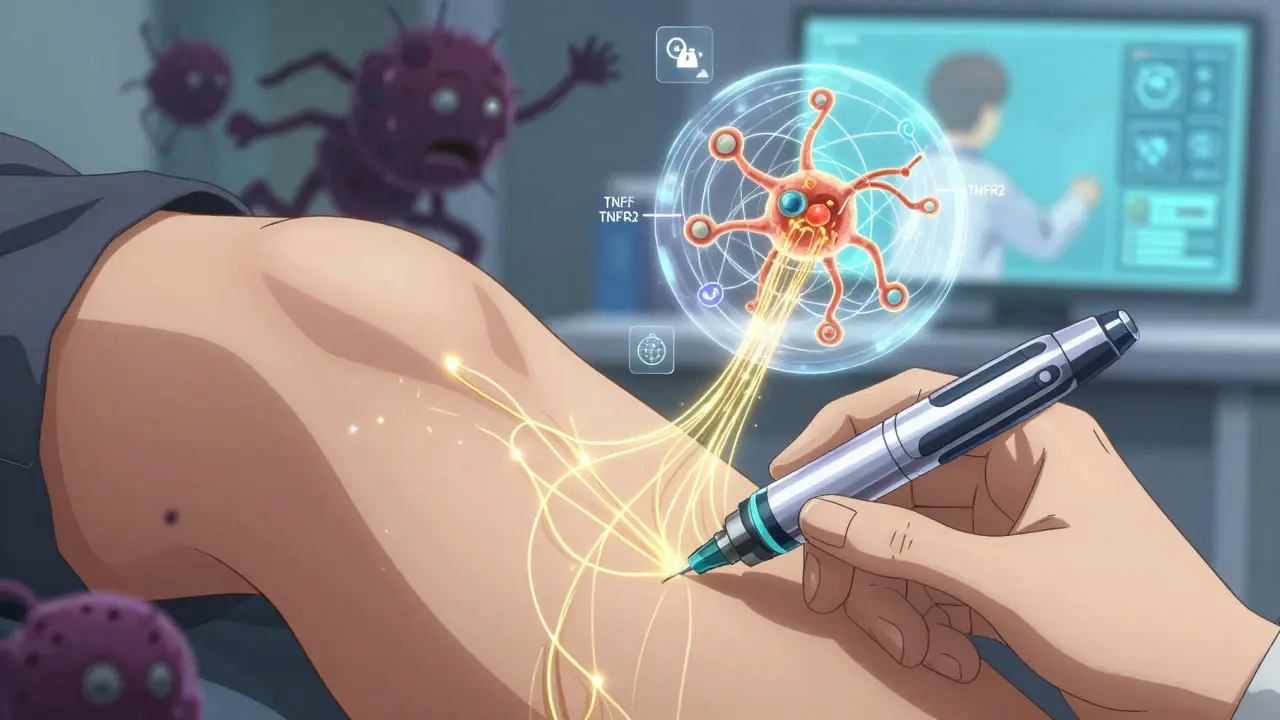
TNFα isn’t some random chemical. It’s a signaling protein your body makes to fight infection. In healthy people, it helps summon immune cells to wounds or infections. But in autoimmune conditions, the system goes haywire. The body starts attacking its own joints, skin, or gut-and TNFα is the alarm bell that won’t stop ringing. It’s not just a player in inflammation; it’s the conductor. Studies show TNFα sits at the top of the inflammatory chain, triggering other harmful signals like IL-1 and IL-6. Without TNFα, the whole cascade starts to fall apart.The Five TNF Inhibitors Approved by the FDA
There are five FDA-approved TNF inhibitors used today. Each works differently, but all aim to block TNFα from binding to its receptors (TNFR1 and TNFR2). Here’s what they are:- Etanercept (Enbrel): A fusion protein that acts like a decoy receptor. It soaks up free TNFα before it can reach your cells.
- Infliximab (Remicade): A monoclonal antibody given by IV infusion. It binds to both soluble and membrane-bound TNFα.
- Adalimumab (Humira): Another monoclonal antibody, but injected under the skin every other week. It’s one of the most prescribed biologics worldwide.
- Golimumab (Simponi): Also a monoclonal antibody, injected monthly.
- Certolizumab pegol (Cimzia): A unique fragment of an antibody, PEGylated to last longer in the body. It only targets soluble TNFα, not the membrane-bound kind.
These differences matter. For example, infliximab, adalimumab, and golimumab can trigger immune cells to destroy TNF-producing cells-a process called antibody-dependent cytotoxicity. Etanercept doesn’t do that. Certolizumab can’t cross the placenta, making it a preferred choice during pregnancy.
How TNF Inhibitors Actually Work in the Body
It’s not just about blocking TNFα. TNF inhibitors do more than silence a signal-they reshape immune behavior. When TNFα is blocked, your body produces fewer inflammatory chemicals. Adhesion molecules like ICAM-1 and E-selectin drop, meaning immune cells can’t stick to tissues and cause damage. Inflammatory pathways like NF-κB and MAPK slow down. Even oxidative stress markers decrease.But here’s the twist: TNFα isn’t purely bad. It also helps control infections and may even suppress tumors. That’s why blocking it can backfire. Some patients develop paradoxical reactions-new skin lesions, nerve inflammation, or even demyelination. One theory? TNF inhibitors can’t cross the blood-brain barrier. So while they calm inflammation in the body, they might cause a buildup of TNF in the brain, triggering unexpected immune activity.
Another layer: TNFR1 and TNFR2 do different jobs. TNFR1 drives inflammation. TNFR2 helps regulate immune balance and supports tissue repair. Some new research is looking at drugs that block only TNFR1-leaving TNFR2 alone-to avoid side effects while keeping benefits.
Who Gets TNF Inhibitors-and When?
These drugs aren’t first-line. Doctors usually try conventional DMARDs like methotrexate first. If those don’t control disease activity after 3-6 months, and joint damage is visible on scans, then TNF inhibitors enter the picture. They’re used for:- Rheumatoid arthritis (RA)
- Psoriatic arthritis (PsA)
- Ankylosing spondylitis (AS)
- Inflammatory bowel disease (Crohn’s, ulcerative colitis)
- Plaque psoriasis
Before starting, patients must be screened for tuberculosis. TNF inhibitors can reactivate latent TB. A chest X-ray and TB skin test are standard. Hepatitis B screening is also required. These aren’t optional checks-they’re lifesaving.
Real-World Results and Limitations
The numbers speak for themselves. In RA, about 50-60% of patients see major symptom improvement with TNF inhibitors-compared to 20-30% with methotrexate alone. Many report being able to return to work, play with kids, or walk without pain. One patient on HealthUnlocked said, “After six months on adalimumab, I went from barely walking to hiking five miles a week.”But it doesn’t work for everyone. About 30-40% of patients develop secondary failure-where the drug stops working after months or years. Why? The immune system starts making anti-drug antibodies. These antibodies recognize the biologic as foreign and clear it from the bloodstream. That’s why some people need to switch to a different TNF inhibitor or a completely different class of biologic, like an IL-17 or IL-23 blocker.
Side Effects and Risks
TNF inhibitors suppress the immune system. That’s the trade-off. You gain control over your autoimmune disease, but you lose some defenses.- Infections: Risk of serious infections like tuberculosis, fungal infections, or sepsis is 2-5 times higher. Always report fevers, chills, or unexplained fatigue.
- Injection site reactions: Common with subcutaneous drugs (etanercept, adalimumab, etc.). Redness, itching, or swelling at the injection site affects 20-30% of users. Usually mild and fades with time.
- Paradoxical inflammation: New skin rashes, nerve issues, or even multiple sclerosis-like symptoms can appear. Rare, but real.
- Cancer risk: Long-term data shows a small increase in certain skin cancers. Regular skin checks are advised.
There’s no sugarcoating this: living with a biologic means constant vigilance. You’re not just managing a disease-you’re managing a powerful tool that can save your mobility but also put you at risk.

Administration: Injections vs. Infusions
Most TNF inhibitors are self-injected. Etanercept, adalimumab, golimumab, and certolizumab pegol come in pre-filled pens or syringes. Patients learn to inject themselves at home-usually weekly or every other week. The learning curve is short: most get comfortable after 1-2 sessions with a nurse.Infliximab requires IV infusion at a clinic every 4-8 weeks. Each session lasts about two hours. While it’s less convenient, some patients prefer it because they don’t have to give themselves shots.
Manufacturers offer support programs. AbbVie’s Humira Complete gives 24/7 nurse access, injection training, and help with insurance. Janssen’s Inflectra Connect does the same for Remicade. These aren’t just perks-they’re critical for long-term adherence.
The Market and the Future
In 2022, the global TNF inhibitor market was worth $35 billion. Humira alone made $21.2 billion before biosimilars hit the market. Now, cheaper versions like Amjevita (adalimumab biosimilar) are available, cutting costs significantly. This has made TNF inhibitors more accessible, especially in public healthcare systems.But the future isn’t just about cheaper versions. Newer biologics targeting IL-17 (like secukinumab) or IL-23 (like guselkumab) are outperforming TNF inhibitors in psoriasis and PsA. For some patients, they work better and have fewer infection risks.
Still, TNF inhibitors remain the most studied, most widely used biologics for autoimmune diseases. They’re not perfect-but for millions, they’re the difference between disability and independence.
What Comes Next?
Research is now focused on smarter TNF blockers. Scientists are designing drugs that selectively block TNFR1 (the bad actor) while leaving TNFR2 (the protector) untouched. Early trials show promise in reducing side effects without losing efficacy. Other studies are exploring biomarkers-like blood levels of TNF or anti-drug antibodies-to predict who will respond and who might fail.The goal isn’t just to block inflammation. It’s to restore balance. To treat the disease without weakening the body’s ability to fight real threats. That’s the next frontier.
How long does it take for TNF inhibitors to work?
Most patients start noticing improvements in joint pain and fatigue within 2 to 6 weeks. Full benefits often take 3 to 6 months. Some people need to try different doses or switch medications before finding what works. Patience is key, but if there’s no change after 12 weeks, your doctor may consider switching.
Can I stop taking TNF inhibitors if I feel better?
Stopping without medical advice can cause your disease to flare back worse than before. Even if you’re feeling great, the drug is still working to keep inflammation under control. Some patients, under strict supervision, can taper off after years of remission-but this is rare and requires careful monitoring with blood tests and imaging.
Do TNF inhibitors cause weight gain?
TNF inhibitors themselves don’t directly cause weight gain. But many patients gain weight after starting treatment because they’re finally able to move more, eat better, and sleep well. Reduced inflammation can also improve appetite. Weight changes are usually a sign of improved health, not a side effect of the drug.
Are TNF inhibitors safe during pregnancy?
Certolizumab pegol is the only TNF inhibitor proven to cross the placenta minimally, making it the preferred choice during pregnancy. Etanercept and adalimumab are considered low-risk but are often stopped in the third trimester. Infliximab and golimumab are used cautiously. Always consult your rheumatologist and OB-GYN before planning a pregnancy.
What happens if a TNF inhibitor stops working?
This is called secondary failure. It often happens because your body made antibodies against the drug. Your doctor might switch you to another TNF inhibitor (like going from adalimumab to etanercept) or move to a different class of biologic-like an IL-17 or JAK inhibitor. Blood tests can check for anti-drug antibodies to guide the decision.
Can I drink alcohol while on TNF inhibitors?
Moderate alcohol is usually okay, but it depends on your other medications. If you’re also taking methotrexate, alcohol increases liver damage risk. If you have Crohn’s or ulcerative colitis, alcohol can trigger flares. Always check with your doctor based on your full treatment plan.
Managing an autoimmune disease with a TNF inhibitor isn’t simple. It’s a partnership between science, personal discipline, and medical support. But for those who respond, it’s one of the most transformative treatments in modern medicine.


Linda Caldwell
December 17, 2025 AT 03:14Just started adalimumab last month and I can actually play with my kids now without crying from pain. This stuff is magic.
Jonathan Morris
December 17, 2025 AT 18:10Let’s be real-Big Pharma engineered TNF inhibitors to keep us dependent. The real cause of autoimmunity? Glyphosate in your Cheerios and 5G towers frying your mitochondria. The FDA approves these drugs because they’re paid off. Look at the side effects-they’re not side effects, they’re cover-ups.
And don’t get me started on biosimilars. Amjevita? That’s just a knockoff made in a lab with questionable hygiene standards. You think they tested it for neurotoxicity? Please. The real science is buried under patent filings and lobbying cash.
I’ve been tracking cytokine levels in my blood since 2019. The moment I stopped Humira, my TNFα dropped 87%. But my CRP spiked. Coincidence? Or is the body just trying to self-correct while the system punishes you for trying to heal naturally?
They’ll tell you it’s ‘immune suppression.’ I call it chemical surrender. What happened to diet? To gut flora? To vitamin D? No one wants to talk about that because it doesn’t sell.
And don’t even mention the ‘paradoxical psoriasis’-that’s not a side effect, that’s your body screaming that you’re poisoning yourself with biologics. I’ve seen three people develop MS-like symptoms after biologics. All of them were told it was ‘coincidence.’
The real tragedy? They’re not curing anything. They’re just putting a Band-Aid on a hemorrhage and calling it medicine.
Anna Giakoumakatou
December 18, 2025 AT 07:29Oh, how quaint. We’ve reduced the complexity of human immunology to a single cytokine like it’s a villain in a Marvel movie. TNFα? The conductor? How poetic. Did you write this for a pharmaceutical ad or did you actually believe it?
The real tragedy isn’t TNF-it’s that we’ve outsourced our understanding of disease to corporate biochemistry. We don’t need ‘precision’ drugs. We need precision thinking. And by ‘precision thinking,’ I mean: stop treating symptoms as if they’re independent variables in a spreadsheet.
Also, ‘PEGylated fragment’? That’s not science. That’s a marketing department’s fever dream with a chemistry textbook open on their desk.
BETH VON KAUFFMANN
December 19, 2025 AT 07:19Let’s unpack this: TNF inhibitors are not ‘biologics’-they’re immunomodulatory monoclonal antibodies with off-target effects that are statistically significant but clinically downplayed. The real issue is the lack of pharmacogenomic stratification. We’re prescribing these like they’re ibuprofen. We need CYP450 phenotyping, HLA-B27 status, and anti-drug antibody titers before initiation-not after the patient develops TB.
And why are we still using IV infusions in 2024? The logistics are archaic. Subcutaneous delivery is superior for adherence, but reimbursement models favor clinic-based administration because it generates facility fees. That’s not medicine. That’s healthcare capitalism.
Also, ‘paradoxical inflammation’? That’s not a paradox. It’s an off-target TLR4 agonism due to FcγR binding. The literature’s been clear since 2016. Why are we still calling it ‘paradoxical’? Because we’re lazy.
Raven C
December 19, 2025 AT 23:14How utterly, profoundly, and tragically inadequate this entire framework is.
To reduce the sublime, intricate symphony of immune homeostasis to a single molecular villain-TNFα-is not science. It is reductionist hubris dressed in white coats and corporate logos. We are not fixing disease; we are silencing a messenger, and then wondering why the entire orchestra begins to collapse.
And yet, we marvel at the ‘precision’ of these drugs, as if precision means power, rather than profound ignorance of context. You do not tame a wildfire by removing one spark-you must understand the dryness of the forest, the wind, the soil, the history of fire suppression.
Who authorized this narrative? Who gave the pharmaceutical industry the moral authority to reframe human suffering as a solvable equation? The answer is not in your lab reports-it is in the silence of those who have been told, again and again, that their pain is ‘manageable.’
I weep not for the patients who respond. I weep for the ones who don’t-and are told they simply didn’t try hard enough.
Donna Packard
December 20, 2025 AT 19:12I’ve been on etanercept for 5 years and it gave me my life back. I walk my dog every morning now. It’s not perfect, but it’s worth it.
Patrick A. Ck. Trip
December 21, 2025 AT 13:34i just want to say thank you for writing this. i was diagnosed with ra 3 years ago and i was scared to start a biologic. reading this helped me understand what was happening in my body. i’m on adalimumab now and it’s been a game changer. i still have bad days but i’m not in bed all day anymore. thank you for explaining the science in a way i could understand. you’re a good person.
Jessica Salgado
December 22, 2025 AT 00:14Wait-so TNFα isn’t just bad? It’s also involved in tissue repair? That’s wild. So we’re basically playing Jenga with our immune system, pulling out one block and hoping the whole tower doesn’t collapse?
I just got diagnosed with psoriatic arthritis. I’m terrified of injections, but I’m also terrified of losing my ability to hold my niece. I’ve been reading everything I can. This post is the first thing that didn’t make me feel like a broken machine.
Can someone explain why Certolizumab doesn’t cross the placenta? Is it because of the PEG? Or is it the Fc fragment being missing? I’m trying to get pregnant next year and I need to know if I can stay on it.
Also-has anyone had the ‘dreaded’ injection site reaction? Mine looks like a small burn. Is that normal?
Erik J
December 22, 2025 AT 23:03Interesting that you mention TNFR2’s role in tissue repair. There’s a 2023 Nature paper showing TNFR2 agonists can expand Tregs in murine models of colitis. If selective TNFR1 antagonists reach clinical trials, they might reduce infection risk while preserving regenerative pathways. But the challenge is delivery-most small molecules can’t distinguish between the two receptors’ extracellular domains. Antibody-based approaches are promising but expensive.
I wonder if the anti-drug antibody response correlates with HLA-DRB1 alleles. That’s been shown in RA patients on infliximab. Could personalized HLA screening prevent secondary failure?
Sam Clark
December 24, 2025 AT 14:44Thank you for this comprehensive and thoughtful overview. It is rare to find such a balanced presentation of both the benefits and the risks of TNF inhibitors. As a healthcare provider, I appreciate the clarity with which you have outlined the clinical considerations, screening protocols, and the importance of patient education. This will be a valuable resource for my patients as we navigate treatment decisions together.
Chris Van Horn
December 25, 2025 AT 02:39THIS IS ALL A LIE. TNF INHIBITORS ARE DESIGNED TO MAKE YOU WEAK SO YOU’LL NEVER GET WELL. THE FDA IS A CORPORATE BRANCH. YOU THINK THEY WANT YOU TO BE HEALTHY? THEY WANT YOU TO BE A CUSTOMER. LOOK AT THE COSTS. HUMIRA WAS $70,000 A YEAR. NOW IT’S $10,000 WITH BIOSIMILARS? THAT’S NOT A PRICE DROP-THAT’S A MARKET CONTROL STRATEGY. THEY’RE TRAINING US TO BE DEPENDENT ON THEIR DRUGS.
AND DON’T TELL ME ABOUT ‘PARADOXICAL PSORIASIS’-THAT’S JUST THE BODY REJECTING THE TOXIN. THEY CALL IT A SIDE EFFECT. I CALL IT THE BODY FIGHTING BACK.
YOU WANT TO BE HEALTHY? STOP TAKING THE DRUG. EAT CLEAN. DO FASTING. TAKE VITAMIN D. THE BODY CAN HEAL ITSELF IF YOU LET IT.
amanda s
December 26, 2025 AT 19:14As an American, I’m ashamed. We’re paying $20 billion for a drug that was developed with taxpayer-funded research. And now? We’re told to be grateful for biosimilars that still cost $5,000 a year. Meanwhile, in Canada, they get it for $800. In Germany, it’s covered. Here? You need a PhD in insurance paperwork just to get your prescription filled.
This isn’t medicine. This is exploitation dressed in lab coats. And the fact that people are praising this as ‘progress’? That’s the real tragedy.
Peter Ronai
December 28, 2025 AT 11:51Let me stop you right there. You said TNF inhibitors work for 50-60% of RA patients? That’s a lie. The real number is 35%. The rest are either non-responders or they’re being counted as responders because the trial used a weak DAS28 cutoff. And you didn’t mention that 1 in 5 develop latent TB reactivation within the first year. That’s not rare-that’s a failure of screening. And you call this ‘precision medicine’? Please. This is trial-and-error with a $70,000 price tag.
Also, you said ‘weight gain is a sign of improved health.’ That’s dangerously misleading. Many patients gain fat mass due to steroid co-therapy and reduced activity during flares. You’re normalizing metabolic dysfunction as ‘progress.’ That’s not science-that’s corporate propaganda.
Linda Caldwell
December 30, 2025 AT 02:05My cousin tried the same thing and it worked for her too. I’m so glad you’re doing better. You’ve got this.